Oxidative stress is a term often thrown around in health and wellness circles, but what does it really mean? At its core, oxidative stress is an electrochemical concept stemming from an imbalance in the rate of electron transfer within our bodies. This imbalance can lead to a cascade of issues affecting everything from our energy levels to our blood pressure. Understanding the root cause—an inappropriate rate of electron flow—is key to addressing and mitigating its effects. This article will explore the electrochemical basis of oxidative stress, its impact on cellular functions, and practical strategies to support your body’s natural antioxidant defenses.
We’ll delve into how electron transfer works in chemical reactions, the roles of oxidation and reduction, and how this all ties into the creation of reactive oxygen species (ROS) and free radicals. Furthermore, we’ll examine the critical role of antioxidants like glutathione and CoQ10 in maintaining redox balance. Finally, we’ll discuss practical steps you can take to enhance your body’s antioxidant production and protect yourself from the harmful effects of oxidative stress. Let’s unravel the complexities of oxidative stress and empower you with the knowledge to take control of your health.
Understanding Electron Transfer and Redox Reactions
The fundamental concept behind oxidative stress lies in electron transfer, a process vital to countless chemical reactions in the body. Electrons, the tiny particles carrying electric currents, are transferred between chemicals during these reactions. When Chemical A transfers electrons to Chemical B, the properties of both chemicals can change. This is where oxidation and reduction come into play.
Oxidation is the loss of electrons, while reduction is the gain of electrons. If Chemical A extracts electrons from Chemical B, Chemical B is said to be oxidized, and Chemical A is the oxidizing agent, getting reduced in the process. A common example is the rusting of steel. Iron in the steel reacts with atmospheric oxygen, where oxygen (electron-deficient) extracts electrons from iron, oxidizing it into iron (III) oxide, or rust.
However, sometimes oxidation can lead to instability. When iron becomes chemically unstable upon oxidation, it may attempt to reclaim lost electrons by breaking up other stable compounds. This results in highly unstable reactive oxygen species (ROS), also known as free radicals. These ROS then propagate further oxidation reactions, creating even more unstable ROS species. This cascade is a central feature of oxidative stress.
As the original article states, iron loses its electrons and becomes oxidised, while oxygen gains electrons and is reduced. Iron (III) oxide is a relatively stable compound that will not undergo further unnecessary oxidation or reduction reactions under ambient conditions.
The Significance of Redox Potential
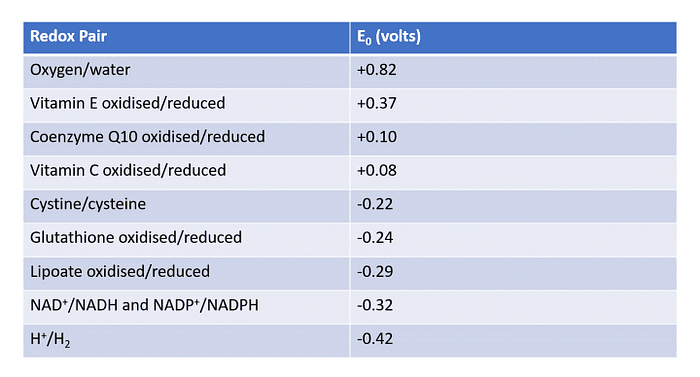
The concept of redox potential is crucial in understanding how electron transfer is regulated in the body. Redox potential (E0) indicates the oxidative capability of a redox pair. A more positive value indicates a higher likelihood of oxidizing something, while a more negative value indicates a greater likelihood of being reduced.
Consider the oxygen/water redox pair with an E0 of +0.82 V and the Coenzyme Q10 (CoQ10) oxidized/reduced redox pair with an E0 of +0.10 V. This data suggests that oxygen will preferentially be reduced into water when in contact with reduced CoQ10, with CoQ10 getting oxidized in the process. This is precisely how CoQ10 functions in human cells, transporting electrons from the electron transport chain to molecular oxygen to produce water.
Having a reduced version of an antioxidant with a lower redox potential helps regenerate oxidized species higher up in the redox chain. Glutathione, often called the master antioxidant, exemplifies this. Our cells produce and regenerate glutathione, enabling it to easily regenerate spent dietary antioxidants like Vitamin C and Vitamin E.
According to the journal paper, different A-B pairings will result in the development of different reduction-oxidation (redox) potentials, as highlighted in Table 1 below:
The Problems with Dysregulated Electron Transfer
Ordinarily, our bodies regulate redox activity effectively. Mitochondria, the powerhouses of our cells, produce ROS as part of their energy generation processes. However, these ROS are kept in check by glutathione antioxidants, produced via the nuclear factor-erythroid 2 p45-related factor 2 (nrf2) transcriptional pathway.
When our cells produce more ROS than glutathione can handle, dietary antioxidants like Vitamin C and Vitamin E act as a second line of defense. However, if even these defenses are overwhelmed, an imbalance occurs, leading to oxidative stress. This is defined as an imbalance between reactive oxygen species and antioxidant reserves, which can alter the structure and function of proteins, lipids, and DNA.</p
Oxidative stress isn’t just a chemical problem; it profoundly impacts biological functions at a cellular level. If cells are subjected to these biochemical stressors, they cannot operate optimally. This cellular dysfunction can manifest in various health issues.</p
As the original author stated, oxidative stress can result in the altered structure and function of proteins, lipids and DNA.</p
The Impact on Blood Pressure Regulation
Consider the example of blood pressure regulation. Endothelial cells lining our blood vessels produce nitric oxide (NO) using the enzyme endothelial nitric oxide synthase (eNOS) and a cofactor called tetrahydrobiopterin (BH4). NO signals blood vessels to dilate, facilitating healthy blood flow.</p
However, oxidative stress disrupts this process in several ways. First, NO can be oxidized into peroxynitrite radicals, which are ineffective in regulating vasodilation. Second, BH4 can be oxidized into dihydrobiopterin (BH2), leading to eNOS decoupling and an inability to produce NO. Consequently, endothelial cells cannot function properly under oxidative stress.</p
With insufficient vasodilation, blood vessels constrict more easily, forcing the heart to pump harder to maintain blood flow. Over time, this can lead to hypertension. Therefore, hypertension can be viewed as a result of impaired cell functions due to oxidative stress.</p
Oxidative stress is a concept that we can bring about on ourselves unknowingly, let’s learn how to keep our bodies in better shape.</p
Strategies for Mitigating Oxidative Stress
To mitigate oxidative stress, it’s essential to support the body’s natural antioxidant production capacities. One key strategy is upregulating the nrf2 pathway, which enhances the production of glutathione and other endogenous antioxidants. Lifestyle and dietary choices can significantly influence nrf2 activity.</p
Key to remember is that when the cellular mitochondria are producing ROS from the energy generation process, the cells do have internal control systems (glutathione and glutathione reductase) to neutralise the ROS.</p
Unless we do things that actually facilitate the antioxidant production capacities in our body, such as upregulating the nrf2 pathway:
Conclusion
Oxidative stress is an electrochemical imbalance that has far-reaching implications for cellular health and overall well-being. Understanding the dynamics of electron transfer, redox potential, and the role of antioxidants is crucial in mitigating its harmful effects. By supporting our body’s natural antioxidant defenses, such as upregulating the nrf2 pathway and making informed dietary choices, we can better protect ourselves from oxidative stress.
Ultimately, being conscious of the factors that contribute to oxidative stress empowers us to make proactive choices that support optimal cellular function. From regulating blood pressure to maintaining energy levels, addressing oxidative stress is a key component of a holistic approach to health. Embrace strategies to enhance your antioxidant production and pave the way for a healthier, more resilient you.
